| The Electrodeposition of Lead Dioxide |
|---|
| |
There have been a number of different baths proposed for the
electrodeposition (plating) of (Beta) Lead Dioxide but the bath that has gained general
acceptance is the bath containing Lead Nitrate. The Lead Nitrate bath plates well
under the widest range of parameters and is easy to make up. There is rather a lot of contradictory results in scientific literature regarding Alpha
and/or Beta Lead Dioxide being deposited from acidic Lead Nitrate baths, see JES, 149 (9), 2002 . High plating current density gives Alpha, low plating current density gives Beta. Beta type Lead Dioxide is desired as the outside layer of Anodes.
The plating process can be represented by the following overall equation:
Pb(NO3)2 + 2H2O =====>> PbO2 + 2HNO3 + H2
( two Electrons are required)
Nitric acid is produced at a rate of two moles of Nitric acid (126 grams) for ever one mole of Lead Dioxide (239.2 grams) deposited with 2 moles (53.604 Ah) of electrons required when plating is proceeding at 100% current efficiency (CE). The rate of Lead Dioxide deposition at 100% CE is 4.462 grams per amp per hour. The build up of Nitrites in the tank causes CE to drop to very low levels if action is not taken to eliminate them.
| The Lead Nitrate Bath |
| Lead Nitrate (to begin)* |
200 to 800g/l | |
| Copper Nitrate |
A few g/l | |
| Nitric acid |
Approx. 8 grams/l (100% Acid) | |
| Anode current density | From 10 to 100mA/cm2
| |
|
Temperature | From 40 to 80°C | |
| |
If the bath is not to have Lead Nitrate crystallizing out as it cools to room temperature,
the Lead Nitrate should be kept below 400 grams per liter.
The solubility of Lead Nitrate in Grams per Liter of water is approx. 360g/l @
0°C, 560g/l @ 20°C & 960g/l @ 60°C.
The solubility in the tank will be a small amount less than this due to the presence of
Nitric acid which suppresses solubility. At low Nitric acid concentrations (good plating conditions) there is little difference.
The density of solutions made from adding 100, 200, 300 and 350 gram amounts of Lead Nitrate to one liter of water are 1.078, 1.157, 1.236 and 1.275 grams per ml respectively. The density figure of 1.157 represents a solution concentration of 193 grams per liter of Lead Nitrate solution. The figure could be used as an approximate indicator of the lower limit of Lead Nitrate concentration for good plating.
Distilled water should be used if possible but clean rain water will do if distilled is not available. The more shortcuts you take the more likely a failure will occur when
the Anode goes into service (or before).
See here for further discussion
regarding plating baths in general and alternative baths that can be used for plating.
See here for a listing of alternative plating baths.
The Lead Nitrate can be make using Lead metal (used for roofing
and is available from builders providers) and Nitric acid.
See Making Lead nitrate and other Lead compounds.
The Copper Nitrate can be make by
reacting an excess of Copper wire with Nitric acid and adding resulting blue liquid to
plating tank.
The function of the Copper Nitrate is to stop Lead metal from being
electro deposited on to the Cathodes of the plating tank and therefor wasting
Lead ions. Quite a lot of Lead will be deposited on to the Cathodes if you are
using a near neutral plating solution. See -discussion regarding plating baths-
(link above).
Current Density on Anode
The amount of Beta or Alpha that is plated can be controlled by current density on the Anode according to one source I have communicated with. High current density (70mA per square cm) giving mainly the Alpha, 40mA per square cm and below giving mainly the Beta. This has been confirmed by X ray studies. See Electrochimica Acta 52 (2006) 786–793 for refs on info and also JES, 149 (9), 2002 . regarding Alpha/Beta from Lead Nitrate baths.
Sometimes a large current density is used at the start of plating in order to create a large density of PbO2 nucleation sights to improve adhesion of the PbO2 to the substrate.
Current Density on Cathode
US patent 2,945,791 states that current density on Cathodes should be at 2X to 3X Anode current density.
The following hardware will also be needed.
The Plating tank can be a glass beaker as it is good to be able to
see the Anode plating. Use a good tank as
you do NOT want it to break and spill toxic Lead Nitrate all over the
place.
Heater for tank.
This can come in the form of an aquarium heater with it's control altered to let it heat to 70°C. A magnetic hot plate stirrer can be used or other system that will heat the solution to 70° approx. Test out your system using water first to see how it is working.
A DC supply capable of putting
out a few Volts and the required plating current is needed. Generally lower plating currents give smoother coats of Lead Dioxide and about 20mA/cm^2 is good. This current density will give Beta Lead Dioxide from the Lead Nitrate tank. For some ideas on power supplies see Power supplies.
When deciding what plating current to use you simply calculate the surface area
that you have to plate (cm squared) and multiply it by the current density (in
mA/cm squared) you want to plate at. This will tell you the amount of mA that you need
to put into the Anode. You simply adjust the Voltage on the supply to give you
that current. If using the battery charger supply in the link above, you simply attach the required
number of flying Leads to the Anode. A constant current source is good for this
type of application. The Anode is connected to the positive lead.
The Cathodes for
the plating tank can be Copper. Don't use Gouging rods, there are reports that some
Gouging rods contain Iron particles. Iron is NOT wanted in the tank.
The Cathodes surround the substrate that you are
plating and three or four should be OK. The surface area of the Cathode(s)
facing the substrate to be plated should be at least half the surface area that
you are plating. Alternatively a spiral of Copper wire can be used surrounding
the substrate or some Copper strips. Distance
between substrate (Anode) and Cathode is not critical and can be of the order of
about an inch or two. There should be no sharp corners or indents on the Anode
to be plated.
Nitric acid and Lead ion concentration control.
Nitric acid is formed as plating progresses. To stop the concentration of Nitric acid from becoming too high you must have a suitable large tank (if using a one tank system) If using a two tank system with a pump, then the total plating solution volume need not be so large. You must add neutral solution to the plating tank at the appropriate rate.
100%
HNO3 g/l | pH |
| 1 | 1.8 |
| 2 | 1.5 |
| 4 | 1.2 |
| 6 | 1.02 |
| 8 | 0.9 |
| 10 | 0.8 |
| 12 | 0.72 |
| 14 | 0.65 |
| 18 | 0.54 |
| 22 | 0.46 |
| 30 | 0.32 |
| 38 | 0.22 |
| 50 | 0.1 |
| 100 | -0.02 |
It would appear from patents etc, that the best concentration of Nitric acid to have in your plating bath is in the region of 6 to 10 grams Nitric acid per liter. The Nitric acid concentration will increase once you start to plate. You will get two moles of Nitric acid forming for every mole of Lead Dioxide that you plate which needs 53.604 Ampere hours of electrons. Thats 126 Grams acid for every 239.2 Grams Lead Dioxide, = 0.527 grams acid per gram Lead Dioxide deposited. An alternative way to describe this is to say that you will get 2.3506 grams of acid forming per hour per Amp. Most amateur Anodes end up getting formed from baths with much more that the ideal concentration of Nitric acid in the plating solution. If using a one tank system (with no addition of Lead Compounds) a large tank is necessary. If you start your plating operation off with 6 grams per liter Nitric acid and you want to avoid the acid concentration going above 12 grams per liter Nitric acid you will need a tank size of 87.8ml per gram Lead Dioxide that you want to deposit.
Figure out how many grams of Lead Dioxide you need to deposit to form your Anode from the projected Anode dimensions minus the substrate volume multiplied by the density of Lead Dioxide (9.37 grams per cc).
For example if you want to deposit 100 grams Lead Dioxide (not a large Anode) and you decide to start off your plating solution at 6 grams per liter Nitric acid and you do not wish for the acid concentration to go above 12 grams per liter, then you need a minimum (one tank system with no adding in of Lead Compounds during plating) of 100 multiplied by 87.8 = 8.8 liters. That's a big tank!! It would appear that adding Lead Compound (with or without a second tank) is the sensible way to go if the acid concentration is to be kept in the most desirable range.
The pH of water and Nitric acid can be calculated from this page. pH values corresponding to Nitric acid amounts per liter of water are given in the table (as per calculator at link) and they correspond to actual measured values of pH when Nitric acid was added to a Lead Nitrate solution of 340 grams per liter. The molarity of 70% HNO3 is 15.6 and it's density is 1.42 g/cc.
The amount of Lead ion per gram of PbO2 is 0.839 grams. The amount of Lead ion per gram of Lead Nitrate (Pb(NO3)2) is 0.6256 grams. Therefor each gram of
deposited Lead Dioxide will use up 1.341 grams Lead Nitrate. The concentration of Lead Nitrate should not be let fall below 200g/liter. If the tank size is large enough to keep the Nitric acid concentration at reasonable levels then Lead Ion depletion of the plating solution will not be a problem. It is advantageous to have the Lead ion and Nitric acid concentration as constant as possible during plating.
When going for a one or two tank system and adding Lead Compound as plating progresses, the total plating solution volume need not be as large as when using a one tank system with no addition of Lead Compound.
If using a one tank system and adding Lead compound you will need to add the amounts in the table below
Each mole of Electrons (26.8 ampere hours) that flows will produce one mole Nitric acid (63 grams), assuming 100% current efficiency, which is equal to 2.35 grams Nitric acid forming per hour per amp.
Another way to look at this is to say that a half mole of Lead Dioxide is plated onto the Anode per 26.8 ampere hours, (119.6 grams Lead Dioxide per 26.8 ampere hours) which is equal to 4.462 grams Lead Dioxide per amp per hour. You need to add Lead compound to replenish the transfered Lead Ions and depending on the Lead compound you are using the weight added will need to equal the molar amounts of Lead Ion being transfered. The table below gives the relevant molecular weights of the Lead compounds that are used and thus the amounts to add to the tank per amp per hour. This amount of addition will keep the extra acid generated neutralized as well. This is the rate at 100% CE.
| Compound |
Grams of compound to add to tank
per amp per hour @100% CE |
| Litharge (molecular weight = 223) |
4.16 |
| Lead Carbonate (molecular weight = 267) |
4.984 |
| Basic Lead Carbonate (molecular weight = 775) |
4.822 |
The addition of Carbonate or Basic Carbonate will cause problems with foaming, bubbles and floating. A slurry of these compounds may have to be made (by adding some of the compound + some neutral plating solution to a well capped container and shaking) in order stop them floating on the surface of the plating solution. Lead Carbonate comes in a large array of molecular weights so check what you have.
It may be possible to have an excess of Litharge at the bottom of a one tank system (if stirring is not used or perhaps intermittent stirring) which will react fairly slowly with the Nitric as it is produced and help keep the Lead ion concentration constant and the Nitric acid concentration in the wanted range. If the Litharge is too finely divided (small particle size) it may react too easily with the Nitric acid and be inclined to keep the pH of the bath too high (concentration of the Nitric acid will be too low). You cannot use Lead Carbonate or Hydroxide or Basic Lead Carbonate in excess in the one tank system as they will keep the pH too high (they react too readily with the Nitric acid formed) and the Carbonate will also cause bubbles/foaming as it reacts with the acid. See here for some observations regarding addition of Lead compounds to the tank.
From US Patent No. 4,130,467, we have Litharge addition rates of 4 grams per amp per hour in one example and 3.5 grams per hour per amp in another example. The additions were made every two hours.
They used a six liter tank in the second example which gives an idea of tank size/deposition rate between each addition of Litharge. It is next to impossible to get a figure for surface area they were plating.
![[DIAGRAM OF PLATING TANK ]](tank.gif)
| When using a two tank system with Lead Dioxide deposition being performed in one tank and an excess of neutralizing Lead compound in the other tank (neutral solution), then you must pump the neutral liquid into the plating tank at the appropriate rate to keep Nitric acid at the desired concentration and keep Lead ion replenished.
The amount you need to pump out of the plating tank into the neutral tank (the same volume will be pumped from the neutral tank into the plating tank) is given by the formula below:
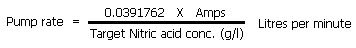 |
| An explanation of this formula is here. At a target of 8g Nitric acid per litre it works out at a pump rate of 4.9ml per Amp per minute. |
The formula assumes 100% CE is being achieved and a somewhat smaller pumping rate may need to be used in practice. It should be noted that no solid Lead compounds should be allowed to come accross from the neutral tank or the pump rate will need to be reduced by a large amount.
The simple arrargement shown may work as a two tank system with a very simple pump based on an aquarium air pump and a bent tube'. Keep the internal diameter of the tube supplying the air fairly big as it may clog up with crystals of Lead Nitrate. Careful of spray, use covers. The air pump could be turned on and off at a suitable frequency and duty cylce using a timer something like this. If an on/off frequency of below 10 minutes or so is chosen then the pumping can be considered to be the equivalent of continous. Perhaps a branch from the main air tube could be used for agigation of the Lead compound in the neutraliser tank or perhaps a second pump and tube. Some plastic tape wrapped around the substrate where there may be variation of plating solution level will give an abrupt known edge to the plating area. The tank should be set up first using a solution of (say) Ammonium Nitrate at a density of approximately 1.3 grams per cc to ascertain pumping rates/times etc. |
There is a useful Microsoft Excell spreadsheet (in a .zip file) here that can be used for calculations regarding the Lead Nitrate plating tank.
Formation of Nitrites
Nitrites are formed at the Cathode as a side reaction and can build up
in the tank.
NO3- + 2e + 2H+ ===> NO2- + H2O
One mole Nitrate + two moles acid + 2 moles electrons ===> One mole Nitrite + 1 mole of water
Then the reaction below starts to occur at the Anode.
NO2- + H2O - 2e - ===> NO3- + 2H+
One mole Nitrite + one mole water - 2 moles electrons ===> One mole Nitrate + 2 moles acid
Since the potential required for the above process (0.80V) is far lower than that
required for the formation of PbO2 (1.46V) , current efficiency will drop as the unwanted reaction if favoured.
The addition of Hydrogen Peroxide to the tank has been used to
counteract this problem by Oxidizing Nitrites back to Nitrates. The build up of Nitrites lowers
current efficiency and will give bad coats of Lead Dioxide. Resting the tank for
a day or two after it has been neutralized will allow Nitrites to convert back
to Nitrates and allow the tank to plate at its best. Bubbling air through the tank will speed up the process. Careful of splashes/spray.
In US Pat. 299464 it is stated that a Sodium Nitrite content of 0.1% will reduce plating CE to 30%. Hydrogen Peroxide (35%) was added slowly over a half hour period to the 1364 liter tank to eliminate Nitrite. Note that they are not making Anode in this patent and the current density is very high on SS that is being plated.
Nitrite testing kits are available for aquarium's and may be useful for detecting Nitrites in the tank.
It may also be possible to test for Nitrites by using a redox indicator like Methylene blue. When Nitrites are present the indicator will be colorless. When eliminated with Hydrogen Peroxide this indicator will turn blue. This indicator may be difficult to see because of the blue colour of Copper Nitrate in the tank as well.
During plating some Copper may be deposited
onto the Cathodes and if the blue colour of the plating solution gets weak, add more Copper Nitrate. There will also be some bubbles on the Anode that are not
welcome but are inevitable. Plating should not be interrupted.
Weigh the Anode substrate before plating so that you will know how much
Lead Dioxide was electro-deposited for calculating plating CE etc.
Keep an eye on evaporation from the tank as it can lower the level of
electrolyte in the tank and leave you with a shorter than expected
Anode. A good way to counteract this problem is to wind on a few layers of insulation tape to the substrate with the bottom edge of the tape at where you want an abrupt end to the LD plate. The substrate in them positioned so that the plating solution surface is in the middle of the taped strip. Some solution volume variation can then be tolerated without giving a feathery edge to the top end of the Lead Dioxide plate.
It is a very good practice to test the tank
using a small piece of Graphite about one inch or so at about
20mA/cm^2 and see that the plating is good. If the tank will not plate
Graphite there is a problem with it. Keep Gouging rods away from the tank.
Ions that are bad news in a plating tank, according to the literature,
are Chloride (US 3,463,707), Iron (US 3,463,707), Cobalt, Selenium and Arsenic.
HIT THE BACK BUTTON ON YOUR BROWSER
![[DIAGRAM OF PLATING TANK ]](tank.gif)